The following are excerpts of a story published by the New York Times:
The Food and Drug Administration said on Friday that it had granted emergency authorization for the first at-home saliva collection kit to test for the coronavirus.
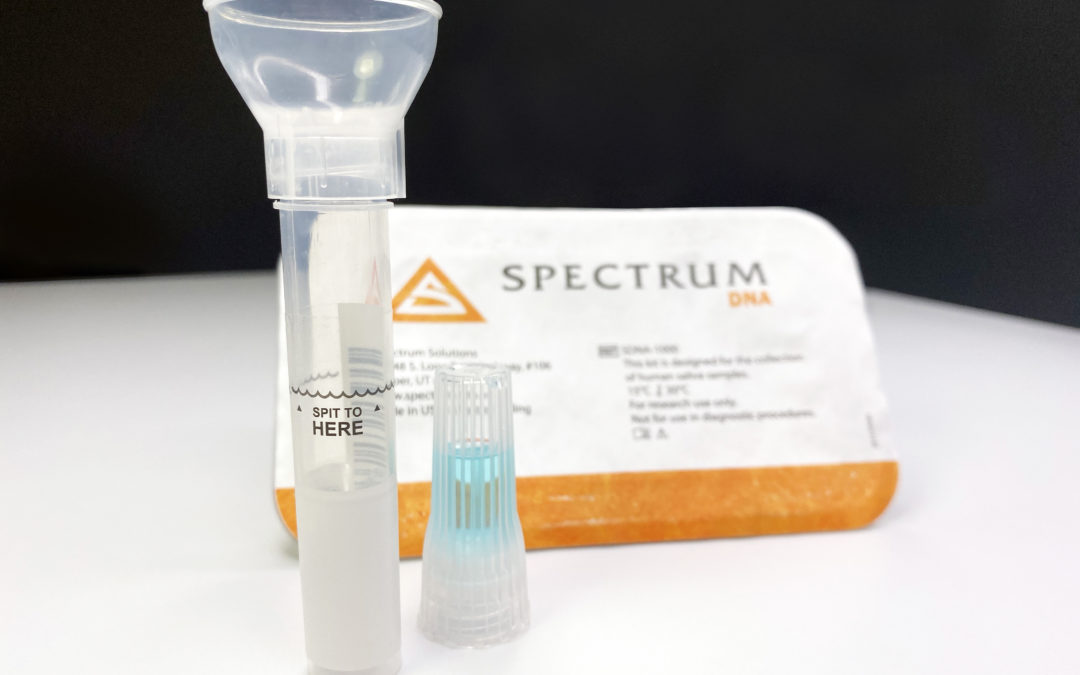
The test kit was developed by a Rutgers University laboratory, called RUCDR Infinite Biologics, in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
Rutgers received FDA permission last month to collect saliva samples from patients at test sites but can now sell the collection kits for individuals to use at home. They must be ordered by a physician.
The FDA said that Rutgers had submitted data showing that testing saliva samples collected by patients themselves, under the observation of a health care provider, was as accurate as testing deep nasal swabs that the health professional had collected from them. The agency also said the spit collection kits should be limited to people who are exhibiting Covid-19 symptoms.
“A patient can open the kit, spit into the tube, put the cap back on and ship it back to our lab,” said Dr. Andrew Brooks, chief operating officer and director of technology development at RUCDR. “We bring the test to the patient, instead of the patient to the test.”
Rutgers has 75,000 of the saliva test kits ready to ship and can process 20,000 tests each day, with a 48-hour turnaround. Dr. Brooks said he expected other labs around the country to adopt it for their own use.
He said that the Centers for Medicare and Medicaid had approved a $100 fee per test, but prices will vary. One company, Vault Health, is now offering telehealth appointments in which a health practitioner supervises the spit test via video. Vault charges $150 per test.
Read the full story in the New York Times.