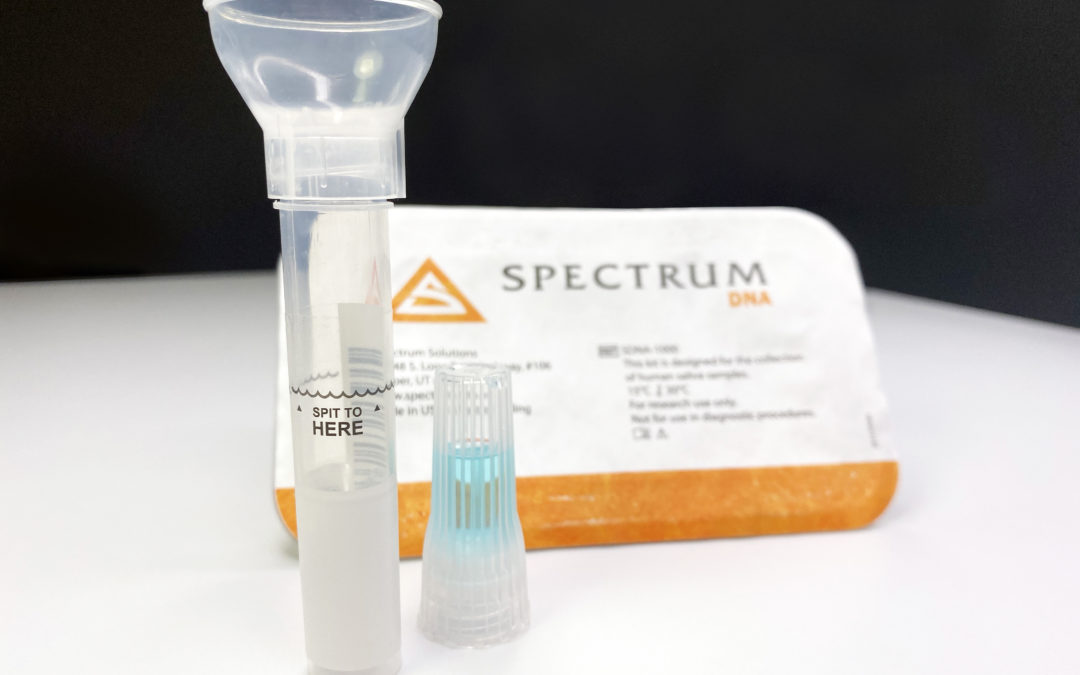
The New York Times reports that the FDA granted emergency authorization for the at-home saliva collection kit that was developed at Rutgers by RUCDR Infinite Biologics. The laboratory’s COO and director of technology development, Andrew Brooks, said Rutgers has 75,000 test kits ready to ship and can process 20,000 tests each day, with a 48-hour turnaround. He expects other labs around the country to adopt it for their own use.